What is a Lead-acid Battery?
The Lead-acid battery is one of the oldest types of rechargeable batteries. These batteries were invented in the year 1859 by the French physicist Gaston Plante.
Despite having a small energy-to-volume ratio and a very low energy-to-weight ratio, its ability to supply high surge contents reveals that the cells have a relatively large power-to-weight ratio.
Lead-acid batteries can be classified as secondary batteries. The chemical reactions that occur in secondary cells are reversible. The reactants that generate an electric current in these batteries (via chemical reactions) can be regenerated by passing a current through the battery (recharging).
The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The method of regenerating active material is called charging.
Sealed Lead Acid Battery
- The sealed lead-acid battery consists of six cells mounted side by side in a single case. The cells are coupled together, and each 2.0V cell adds up to the overall 12.0V capacity of the battery.
- Despite being relatively heavy, lead-acid batteries are still preferred over other lightweight options owing to their ability to deliver large surges of electricity (which is required to start a cold engine in an automobile).
- A completely charged lead-acid battery is made up of a stack of alternating lead oxide electrodes, isolated from each other by layers of porous separators.
- All these parts are placed in a concentrated solution of sulfuric acid. Intercell connectors connect the positive end of one cell to the negative end of the next cell hence the six cells are in series.
Introduction
Lead acid batteries are the most commonly used type of battery in photovoltaic systems. Although lead acid batteries have a low energy density, only moderate efficiency and high maintenance requirements, they also have a long lifetime and low costs compared to other battery types. One of the singular advantages of lead acid batteries is that they are the most commonly used form of battery for most rechargeable battery applications (for example, in starting car engines), and therefore have a well-established established, mature technology base.

Operation of Lead Acid Batteries
A lead acid battery consists of a negative electrode made of spongy or porous lead. The lead is porous to facilitate the formation and dissolution of lead. The positive electrode consists of lead oxide. Both electrodes are immersed in a electrolytic solution of sulfuric acid and water. In case the electrodes come into contact with each other through physical movement of the battery or through changes in thickness of the electrodes, an electrically insulating, but chemically permeable membrane separates the two electrodes. This membrane also prevents electrical shorting through the electrolyte. Lead acid batteries store energy by the reversible chemical reaction shown below.
The overall chemical reaction is:
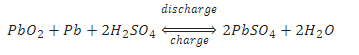
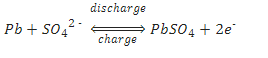
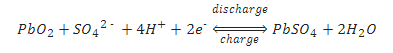
As the above equations show, discharging a battery causes the formation of lead sulfate crystals at both the negative and positive terminals, as well as the release of electrons due to the change in valence charge of the lead. The formation of this lead sulfate uses sulfate from the sulfuric acid electrolyte surrounding the battery. As a result the electrolyte becomes less concentrated. Full discharge would result in both electrodes being covered with lead sulfate and water rather than sulfuric acid surrounding the electrodes. At full discharge the two electrodes are the same material, and there is no chemical potential or voltage between the two electrodes. In practice, however, discharging stops at the cutoff voltage, long before this point. The battery should not therefore be discharged below this voltage.
In between the fully discharged and charged states, a lead acid battery will experience a gradual reduction in the voltage. Voltage level is commonly used to indicate a battery’s state of charge. The dependence of the battery on the battery state of charge is shown in the figure below. If the battery is left at low states of charge for extended periods of time, large lead sulfate crystals can grow, which permanently reduces battery capacity. These larger crystals are unlike the typical porous structure of the lead electrode, and are difficult to convert back into lead.

Voltage of lead acid battery upon charging.
The charging reaction converts the lead sulfate at the negative electrode to lead. At the positive terminal the reaction converts the lead to lead oxide. As a by-product of this reaction, hydrogen is evolved. During the first part of the charging cycle, the conversion of lead sulfate to lead and lead oxide is the dominant reaction. However, as charging proceeds and most of the lead sulfate is converted to either lead or lead dioxide, the charging current electrolyzes the water from the electrolyte and both hydrogen and oxygen gas are evolved, a process known as the “gassing” of the battery. If current is being provided to the battery faster than lead sulfate can be converted, then gassing begins before all the lead sulfate is converted, that is, before the battery is fully charged. Gassing introduces several problems into a lead acid battery. Not only does the gassing of the battery raise safety concerns, due to the explosive nature of the hydrogen produced, but gassing also reduces the water in the battery, which must be manually replaced, introducing a maintenance component into the system. In addition, gassing may cause the shedding of active material from the electrolyte, thereby permanently reducing battery capacity. For these reasons, the battery should not regularly be charged above the voltage which causes gassing. The gassing voltage changes with the charge rate.
Lead sulphate is an insulator, and therefore the way in which lead sulfate forms on the electrodes determined how easily the battery can be discharged.
Characteristics of Lead Acid Batteries
For most renewable energy systems, the most important battery characteristics are the battery lifetime, the depth of discharge and the maintenance requirements of the battery. This set of parameters and their inter-relationship with charging regimes, temperature and age are described below.
Depth of Discharge and Battery Capacity
The depth of discharge in conjunction with the battery capacity is a fundamental parameter in the design of a battery bank for a PV system, as the energy which can be extracted from the battery is found by multiplying the battery capacity by the depth of discharge. Batteries are rated either as deep-cycle or shallow-cycle batteries. A deep-cycle battery will have depth of discharge greater than 50%, and may go as high as 80%. To achieve the same useable capacity, a shallow-cycle battery bank must have a larger capacity than a deep-cycle battery bank.
In addition to the depth of discharge and rated battery capacity, the instantaneous or available battery capacity is strongly affected by the discharge rate of the battery and the operating temperature of the battery. Battery capacity falls by about 1% per degree below about 20°C. However, high temperatures are not ideal for batteries either as these accelerate aging, self-discharge and electrolyte usage. The graph below shows the impact of battery temperature and discharge rate on the capacity of the battery.
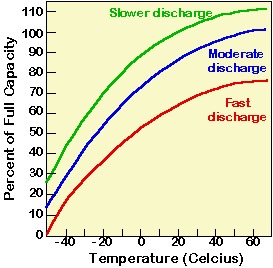
Figure: Relationship between battery capacity, temperature and discharge rate.
Battery Lifetime
Over time, battery capacity degrades due to sulfation of the battery and shedding of active material. The degradation of battery capacity depends most strongly on the interrelationship between the following parameters:
- the charging/discharging regime which the battery has experienced
- the DOD of the battery over its life
- its exposure to prolonged periods of low discharge
- the average temperature of the battery over its lifetime
The following graph shows the evolution of battery function as number of cycles and depth of discharge for a shallow-cycle lead acid battery. A deep-cycle lead acid battery should be able to maintain a cycle life of more than 1,000 even at DOD over 50%.
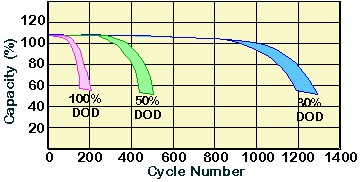
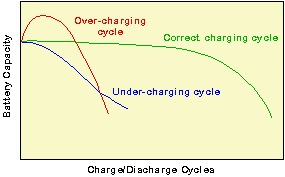
In addition to the DOD, the charging regime also plays an important part in determining battery lifetime. Overcharging or undercharging the battery results in either the shedding of active material or the sulfation of the battery, thus greatly reducing battery life.
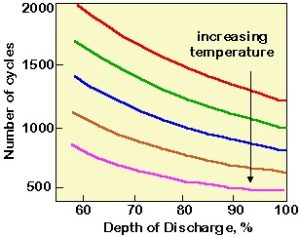
The final impact on battery charging relates to the temperature of the battery. Although the capacity of a lead acid battery is reduced at low temperature operation, high temperature operation increases the aging rate of the battery.
Constant current discharge curves for a 550 Ah lead acid battery at different discharge rates, with a limiting voltage of 1.85V per cell (Mack, 1979). Longer discharge times give higher battery capacities.
Maintenance Requirements
The production and escape of hydrogen and oxygen gas from a battery causes water loss and water must be regularly replaced in lead acid batteries. Other components of a battery system do not require maintenance as regularly, so water loss can be a significant problem. If the system is in a remote location, checking water loss can add to costs. Maintenance-free batteries limit the need for regular attention by preventing or reducing the amount of gas which escapes the battery. However, due to the corrosive nature the elecrolyte, all batteries to some extent introduce an additional maintenance component into a PV system.
Battery Efficiency
Lead acid batteries typically have coulombic efficiencies of 85% and energy efficiencies in the order of 70%.
Lead Acid Battery Configurations
Depending on which one of the above problems is of most concern for a particular application, appropriate modifications to the basic battery configuration improve battery performance. For renewable energy applications, the above problems will impact the depth of discharge, the battery lifetime and the maintenance requirements. The changes to the battery typically involve modification in one of the three basic areas:
- changes to the electrode composition and geometry
- changes to the electrolyte solution
- modifications to the battery housing or terminals to prevent or reduce the escape of generated hydrogen gas.
Special Considerations for Lead Acid Batteries
Flooded lead acid batteries are characterised by deep cycles and long lifetimes. However, flooded batteries require periodic maintenance. Not only must the level of water in the electrolyte be regularly monitored by measuring its specific gravity, but these batteries also require “boost charging”.
Boost Charging
Boost or equalization charging involves short periodic overcharging, which releases gas and mixes the electrolyte, thus preventing stratification of the electrolyte in the battery. In addition, boost charging also assists in keeping all batteries at the same capacity. For example, if one battery develops a higher internal series resistance than other batteries, then the lower SR battery will consistently be undercharged during a normal charging regime due to the voltage drop across the series resistance. However, if the batteries are charged at a higher voltage, then this allows all batteries to become fully charged.
Specific Gravity (SG)
A flooded battery is subject to water loss from the electrolyte due to the evolution of hydrogen and oxygen gas. The specific gravity of the electrolyte, which can be measured with a hydrometer, will indicate the need to add water to the batteries if the batteries are fully charged. Alternately, a hydrometer will accurately indicate the SOC of the battery if it is known that the water level is correct. SG is periodically measured after boost charging to insure that the battery has sufficient water in the electrolyte. The SG of the battery should be provided by the manufacturer.
Special Considerations for Gelled, Sealed Lead Acid Batteries
Gelled or AGM lead acid batteries (which are typically sealed or valve regulated) have several potential advantages:
- they can be deep cycled while retaining battery life
- they do not need boost charging
- they require lower maintenance.
However, these batteries typically require a more precise and lower voltage charging regime. The lower voltage charging regime is due to the use of lead-calcium electrodes to minimise gassing, but a more precise charging regime is required to minimise gassing from the battery. In addition, these batteries may be more sensitive to temperature variations, particularly if the charging regime does not compensate for temperature or is not designed for these types of batteries.
Failure Modes for Lead Acid Batteries
The battery for a PV system will be rated as a certain number of cycles at a particular DOD, charging regime and temperature. However, batteries may experience either a premature loss in capacity or a sudden failure for a variety of reasons. Sudden failure may be caused by the battery internally short-circuiting due to the failure of the electrical separator within the battery. A short circuit in the battery will reduce the voltage and capacity from the overall battery bank, particularly if sections of the battery are connected in parallel, and will also lead to other potential problems such as overcharging of the remaining batteries. The battery may also fail as an open circuit (that is, there may be a gradual increase in the internal series resistance), and any batteries connected in series with this battery will also be affected. Freezing the battery, depending on the type of lead acid battery used, may also cause irreversible failure of the battery.
The gradual decline in capacity may be worsened by inappropriate operation, particularly by degrading the DOD. However, the operation of one part of the battery bank under different conditions to another will also lead to a reduction in overall capacity and an increase in the likelihood of battery failure. Batteries may be unintentionally operated under different regimes due either to temperature variations or to the failure of a battery in one battery string leading to unequal charging and discharging in the string.
Installation
Battery installation should be conducted in accordance with the relevant standard in the country in which they are being installed. At present, there are Australian standards AS3011 & AS2676 for battery installation. There is also a draft standard for batteries for RAPS applications which will eventually become an Australian standard.
Among other factors to be considered in the installation of a battery system are the ventilation required for a particular type of battery bank, the grounding conditions on which the battery bank is to be placed, and provisions taken to ensure the safety of those who may have access to the battery bank. In addition, when installing the battery bank care must be taken to ensure that the battery temperature will fall within the allowable operating conditions of the battery and that the temperature of the batteries in a larger battery bank are at the same temperatures. Batteries in very cold conditions are subject to freezing at low states of charge, so that the battery will be more likely to be in a low state of charge in winter. To prevent this, the battery bank may be buried underground. Batteries regularly exposed to high operating temperatures may also suffer a reduced lifetime.

Safety
Batteries are potentially dangerous and users should be aware of three main hazards: The sulfuric acid in the electrolyte is corrosive. Protective clothing in addition to foot and eye protection are essential when working with batteries.
Batteries have a high current generating capability. If a metal object is accidentally placed across the terminals of a battery, high currents can flow through this object. The presence of unnecessary metal objects (e.g. jewellery) should be minimised when working with batteries and tools should have insulated handles.
Explosion hazards due to evolution of hydrogen and oxygen gas. During charging, particularly overcharging, some batteries, including most batteries used in PV systems, may evolve a potentially explosive mixture of hydrogen and oxygen gas. To reduce the risk of explosion, ventilation is used to prevent the buildup of these gasses and potential ignition sources (i.e. circuits which may generate sparks or arcs) are eliminated from the battery enclosure.
Maintenance
Batteries introduce a periodic maintenance component into a PV system. All batteries, including “maintenance free” batteries require a maintenance schedule which should ensure that:
- the battery terminals are not corroded
- the battery connections are tight
- the battery housing should be free of cracks and corrosion.
Flooded batteries require extra and more frequent maintenance. For flooded batteries, the level of electrolyte and the specific gravity of the electrolyte for each battery needs to be checked regularly. Checking the specific gravity of a battery by using a hydrometer should be carried out at least 15 minutes after an equalisation or boost charge. Only distilled water should be added to batteries. Tap water contains minerals which may damage the battery electrodes.
Battery Disposal and Recycling
The lead in a lead acid battery presents an environmental hazard if it is not properly disposed of. Lead acid batteries should be recycled so that the lead can be recovered without causing environmental damage.
Electrode Materials and Configuration
The materials from which the electrodes are made have a major affect on the battery chemistry, and hence affect the battery voltage and its charging and discharging characteristics. The geometry of the electrode determines the internal series resistance and the charging and discharging rate.
Plate Material
The basic anode and cathode materials in a lead acid battery are lead and lead dixodie (PbO2). The lead electrode is in the form of sponge lead. Sponge lead is desirable as it is very porous, and therefore the surface area between the lead and the sulfic acid electrolyte is very large. The addition of small amounts of other elements to the lead electrode to form lead alloys can reduce several of the disadvantages associated with the lead. The main types of electrodes used are lead/antimony (using several percent antimony), lead/calcium alloys, and lead/antimony/calcium alloys.
Antimony lead alloy batteries have several advantages over pure lead electrodes. These advantages include: the lower cost of lead/antimony; the increased strength of the lead/antimony electrode; and the ability to be deeply discharged for short period of time. However, lead/antimony alloys are prone to sulfation and should not be left at low states of charge for extended periods of time. I addition, lead/antimony alloys increase the gassing of the battery during charging leading to high levels of water loss. Since the water must be added to these batteries, they have higher maintenance. Furthermore, lead/antimony batteries have a high discharge rate and a short lifetime. These problems (xx- check if both problems are caused by plating)) are caused by the dissolution of antimony from one electrode and its deposition or plating on the other electrode. (xx the increased adhesion of PbO2 xx)
Lead calcium batteries are an intermediate cost technology. Like antimony, calcium also adds strength to the lead of the negative electrode, but unlike antimony, the addition of calcium reduces the gassing of the battery and also produces a lower self-discharge rate. However, lead calcium batteries should not be deeply discharged. Consequently, these types of batteries may be considered “maintenance-free”, but are only shallow cycle batteries.
Adding antimony as well as calcium to the electrodes provides some of the advantages of both antimony and lead, but at an increased cost. Deep discharge batteries such as these can also have a high lifetime. Furthermore, trace amounts of other materials can be added to the electrodes to increase battery performance.
Electrode Configuration
In addition to the material used to make the electrode plates, the physical configuration of the electrodes also has an impact on the charging and discharging rates and on the lifetime. Thin plates will allow faster charging and discharging, but are less robust and more prone to shedding of material from the plates. As high charging or discharging currents are not typically a required feature of batteries for renewable energy systems, thicker plates can be used, which have lower charge and discharge times, but also have longer lifetimes.
Battery Housing
In an open, flooded battery, any gas which is generated can escape to the atmosphere, causing both safety and maintenance problems. A sealed lead acid (SLA), valve-regulated lead acid (VRLA) or recombining lead acid battery prevent the loss of water from the electrolyte by preventing or minimizing the escape of hydrogen gas from the battery. In a sealed lead acid (SLA) battery, the hydrogen does not escape into the atmosphere but rather moves or migrates to the other electrode where it recombines (possibly assisted by a catalytic conversion process) to form water. Rather than being completely sealed, these batteries include a pressure vent to prevent the build-up of excess pressure in the battery. Sealed batteries require stringent charging controls to prevent the build-up of hydrogen faster than it can recombine, but they require less maintenance than open batteries.
Valve regulated lead acid (VRLA) batteries are similar in concept to sealed lead acid (SLA) batteries except that the valves are expected to release some hydrogen near full charge. SLA or VRLA batteries typically have additional design features such as the use of gelled electrolytes and the use of lead calcium plates to keep the evolution of hydrogen gas to a minimum.
Types of Lead Acid Batteries
Despite the range in battery types and applications, the characteristics particularly important in PV applications are the maintenance requirements of the battery and the ability to deep charge a battery while maintaining a long lifetime. To promote long cycle life with deep discharge, deep cycle batteries may be either of the open-flooded type, with an excess of electrolytic solution and thick plates, or of the immobilized electrolytic type. Sealed gelled batteries may be rated as deep cycle batteries, but they will usually withstand fewer cycles and lower discharges than the specially designed flooded plate or AGM batteries. Shallow-cycle batteries typically use thinner plates made from lead calcium alloys and do not typically have a depth of discharge above 25%.
Batteries for PV or remote area power supplies (RAPS)
The stringent requirements for batteries used in photovoltaic systems have prompted several manufacturers to make batteries specifically designed for PV or other remote power systems. The batteries most commonly used in stand-alone photovoltaic systems are either deep-cycle lead acid types, or shallower cycle maintenance-free batteries. Deep-cycle batteries may be open flooded batteries (which are not maintenance-free) or captive electrolyte AGM batteries which are maintenance-free (but which do require care in regulator selection). Special shallow-cycle maintenance-free batteries that withstand infrequent discharging may also be used in PV applications, and provided that the battery bank is appropriately designed, never require a DOD of more than 25%. A long-life battery in an appropriately designed PV system with correct maintenance can last up to 15 years, but the use of batteries which are not designed for long service life, or conditions in a PV system, or are part of a poor system design can lead to a battery bank which fails after only a few years.
Several other types of specific purpose batteries are available and these are described below.
Starting, lighting ignition batteries (SLI). These batteries are used in automotive applications and have high discharge and charge rates. Most often they use electrode plates strengthened with either lead antimony in a flooded configuration, or lead calcium in a sealed configuration. These batteries have a good life under shallow-cycle conditions, but have very poor lifetime under deep cycling. SLI batteries should not be used in a PV system since their characteristics are not optimized for use in a renewable energy system because lifetime in a PV system is so low.
Traction or motive power batteries. Traction or motive batteries are used to provide electric power for small transport vehicles such as golf carts. Compared to SLI batteries, they are designed to have a greater ability to be deep-cycled while still maintaining a long lifetime. Although this feature makes them more suited to a PV system than one which uses SLI batteries, motive power batteries should not be used in any PV systems since their self discharge rate is very high due to the use of lead antimony electrodes. A high self discharge rate will effectively cause high power losses from the battery and make the overall PV system inefficient unless the batteries experience large DOD on a daily basis. The ability of these batteries to withstand deep cycling is also far below that of a true deep-cycle battery. Therefore, these batteries are not suited to PV systems.
RV or marine batteries. These batteries are typically a compromise between SLI batteries, traction batteries and true deep-cycle batteries. Although they are not recommended, both motive and marine batteries are used in some small PV systems. The lifetime of such batteries will be restricted to a few years at best, so that the economics of battery replacement mean that such batteries are typically not a long-term cost effective option.
Stationary batteries. Stationary batteries are often used for emergency power or uninterruptable power supply applications. They are shallow-cycle batteries intended to remain close to fully charged for the majority of their lifetime with only occasional deep discharges. They may be used in PV systems if the battery bank is sized so that it never falls below a DOD of between 10% and 25%.
Deep-cycle Batteries. Deep-cycle batteries should be able to maintain a cycle life of several thousand cycles under high DOD (80% or more). Wide differences in cycle performance may be experienced with two types of deep cycle batteries and therefore the cycle life and DOD of various deep-cycle batteries should be compared.
Potential Problems with Lead Acid Batteries
A lead acid battery consists of electrodes of lead oxide and lead are immersed in a solution of weak sulfuric acid. Potential problems encountered in lead acid batteries include:
Gassing: Evolution of hydrogen and oxygen gas. Gassing of the battery leads to safety problems and to water loss from the electrolyte. The water loss increases the maintenance requirements of the battery since the water must periodically be checked and replaced.
Damage to the electrodes. The lead at the negative electrode is soft and easily damaged, particularly in applications in which the battery may experience continuous or vigorous movement.
Stratification of the electrolyte. Sulfuric acid is a heavy, viscous liquid. As the battery discharges, the concentration of the sulfuric acid in the elecotrolyte is reduced, while during charging the sulfiric acid concentratin increases. This cyclicing of sulfuric acid concentration may lead to stratification of the electrolyte, where the heavier sulfuric acid remains at the bottom of the battery, while the less concentrated solution, water, remains near the top. The close proximity of the electrode plates within the battery means that physical shaking does not mix the sulfuric acid and water. However, controlled gassing of the electrolyte encourages water and sulfuric acid to mix, but must be carefully controlled to avoid problems of safety and water loss. Periodic but infrequent gassing of the battery to prevent or reverse electrolyte stratification is required in most lead acid batteries in a process referred to as “boost” charging.
Sulfation of the battery. At low states of charge, large lead sulfate crystals may grow on the lead electrode as opposed to the finely grained material which is normally produced on the electrodes. Lead sulphate is an insulating material.
Spillage of the sulfuric acid. If sulfuric acid leaks from the battery housing it poses a serious safety risk. Gelling or immobilizing the liquid sulfuric acid reduces the possibility of sulfuric acid spills.
Freezing of the battery at low discharge levels. If the battery is at a low discharge level following the conversion of the whole electrolyte to water, then the freezing point of the electrolyte also drops.
Loss of active material from the electrodes. The loss of active material from the electrodes can occur via several processes. One process that can cause a permanent loss of capacity is the flaking off of the active material due to volumetric changes between xxx and lead sulphate. In addition, xxx. Improper charging conditions and gassing can cause shedding of active material from the electrodes, leading to a permanent loss in capacity.
Depending on which one of the above problems is of most concern for a particular application, appropriate modifications to the basic battery configuration improve battery performance. For renewable energy applications, the above problems will impact the depth of discharge, the battery lifetime and the maintenance requirements. The changes to the battery typically involve modification in one of the three basic areas:
- changes to the electrode composition and geometry
- changes to the electrolyte solution
- modifications to the battery housing or terminals to prevent or reduce the escape of generated hydrogen gas.
Corrosion of terminals
Corrosion consists of a set or reduction/oxidation regions in which both the reactions take place at the same electrode. For a battery system, corrosion leads to several detrimental effects. One effect is that it converts a metallic electrode to a metal oxide.
Self-Discharge
All chemical reactions proceed in both the forward and reverse direction. In order for the reverse reaction to proceed, the reactants must gain enough energy to overcome the electrochemical difference between the reactants and the products and also the overvoltage. Usually in battery systems, the probability of the reverse reaction occurring is small, since there are few molecules with a large enough energy. Although small, however, there are some particles that do have sufficient energy. In a charged battery, a process exists by which the battery can be discharged even in the absence of a load connected to the battery. The amount a battery discharges upon standing is known as self-discharge. Self-discharge increases as temperature increases because a greater fraction of products will have enough energy for the reaction to proceed in the reverse direction.
An ideal set of chemical reactions for a battery would be one in which there is a large chemical potential which releases a large number of electrons, has a low overvoltage, spontaneously proceeds in only one direction and is the only chemical reaction which can occur. However, in practice, there are several effects that degrade battery performance, due to unwanted chemical reactions, to effects such as the change in phase of volume of the reactants or products and also to the physical movement of reactants and products within the battery.
Change in form of materials
While undergoing chemical reactions, many materials undergo a change either in phase, or if they stay in the same phase, the volume, density of the material may be altered by the chemical reaction. Finally, the materials used in the battery, primarily the anode and cathode, may change their crystallinity or surface structure, which will in turn affect the reactions in the battery. Many components in redox reactions undergo a change in phase during either oxidation or reduction. For example, in the lead acid battery, sulfate ions changes from being in solid form (as lead sulfate) to being in solutions (as sulfuric acid). If the lead sulfate recrystallizes anywhere but the anode or cathode, then this material is lost to the battery system. During charging, only materials connected to the anode and cathode can participate in electron exchange, and therefore if the material is not touching the anode or cathode, then it can no longer be recharged. The formation of a gaseous phase in a battery also presents special problems. First of all, the gaseous phase will usually have a larger volume that the initial reactants, thus giving rise to a change in pressure in the battery. Secondly, if the intended products are in the gaseous change, they must be confined to the anode and cathode, or they will not be able to be charged.
A change in volume will also usually be detrimental in battery operation.
Modifications to the electrolyte
A standard “flooded” lead acid battery has the electrodes immersed in liquid sulfuric acid. Several modifications to the electrolyte are used to improve battery performance in one of several areas. The key parameters of the electrolyte which control the performance of the battery are the volume and concentration of the electrolyte and forming a ‘captive’ electrolyte.
Electrolyte Volume and Concentration
Changes in the volume of the electrolyte can be used to improve the robustness of a battery. Increasing the volume of an electrolyte makes the battery less sensitive to water losses, and hence makes regular maintenance less critical. Adding to the volume of the battery will also increase its weigth and reduce the energy density of the battery.
Captive Electrolyte Lead Acid Batteries
In ‘captive’ electrolyte batteries, the sulfuric acid is immobilised by either ‘gelling’ the sulfuric acid or by using an ‘absorptive glass mat’. Both have lower gassing compared to a flooded lead acid battery and are consequently often found in “maintenance-free” sealed lead acid batteries.
Gelling. In a “gelled” lead acid battery, the electrolyte may be immobilized by gelling the sulfuric acid using silica gel. The gelled electrolyte has an advantage in that gassing is reduced, and consequently, the batteries are low-maintenance. In addition, stratification of the electrolyte does not occur with gelled batteries and therefore boost charging is not required, and because the electrolyte is gelled, the chances of spilling sulfuric acid are also reduced. However, in order to further reduce gassing, these “gel-cell” batteries also typically use lead calcium plates, making them unsuited to deep discharge applications. A further drawback is that the charging conditions of a gelled lead acid battery must be more carefully controlled to prevent overcharging and damage to the battery.

Absorbative Glass Matting. A second technology which can be used to immobilize the sulfuric acid is “absorptive glass mat” or AGM batteries. In an AGM battery, the sulfuric acid is absorbed in a fiberglass mat which is placed between the electrodes plates. AGM batteries have numerous advantages including the ability to be deeply discharged without affecting lifetime, allowing high rates of charge/discharges and an extended temperature range for operation. The key disadvantage with these batteries is their need for more carefully controlled charging regimes and their higher initial cost.
Scooter Batteries Related Products:
Related Products Application: