What is a Lead-acid Battery?
The Lead acid battery is one of the oldest types of rechargeable batteries. These batteries were invented in the year 1859 by the French physicist Gaston Plante.
Lead-acid batteries can be classified as secondary batteries. The chemical reactions that occur in secondary cells are reversible. The reactants that generate an electric current in these batteries (via chemical reactions) can be regenerated by passing a current through the battery (recharging).
The chemical process of extracting current from a secondary battery (forward reaction) is called discharging. The method of regenerating active material is called charging.
Sealed Lead Acid Battery
- The sealed lead-acid battery consists of six cells mounted side by side in a single case. The cells are coupled together, and each 2.0V cell adds up to the overall 12.0V capacity of the battery.
- Despite being relatively heavy, lead-acid batteries are still preferred over other lightweight options owing to their ability to deliver large surges of electricity (which is required to start a cold engine in an automobile).
- A completely charged lead-acid battery is made up of a stack of alternating lead oxide electrodes, isolated from each other by layers of porous separators.
- All these parts are placed in a concentrated solution of sulfuric acid. Intercell connectors connect the positive end of one cell to the negative end of the next cell hence the six cells are in series.
Despite having a small energy-to-volume ratio and a very low energy-to-weight ratio, its ability to supply high surge contents reveals that the cells have a relatively large power-to-weight ratio.
Since you’re reading this, you obviously have some questions about lead acid battery. For instance, how does a lead-acid battery work? For that matter, what exactly is a lead-acid battery?
Are these batteries still efficient enough for certain uses or are they obsolete? How do they compare to other batteries?
Fortunately, we have all the answers you’ve been looking for right here in this guide. To start with, we can assure you that these batteries are not obsolete. There are, in fact, many applications in which it’s ideal to use lead-acid batteries.
We’ll explain this in more detail below. We also provide a comprehensive explanation about what a lead-acid battery is and how it works. Read on to learn all there is to know about lead-acid batteries.
What Exactly Is a Lead Acid Battery?
A lead-acid battery is a rechargeable battery that uses lead and sulphuric acid to function. The lead is submerged into the sulphuric acid to allow a controlled chemical reaction.
This chemical reaction is what causes the battery to produce electricity. Then, this reaction is reversed to recharge the battery.
Believe it or not, this technology is over 100 years old. However, it has been improved upon since its invention in 1859 and it now works more efficiently.
How Does a Lead Acid Battery Work?
To put it simply, the battery’s electrical charge is generated when the sulphate in the sulphuric acid becomes bonded to the lead. The electrical charge is replenished by reversing this reaction. That is, the sulphate goes back into the sulphuric acid and, thus, the battery is recharged.
Now, obviously, there’s a finite amount of sulphate ions in the acid. And the available surface area of the lead it bonds to is limited, too. So, as the sulphate is depleted, the charge becomes weaker.
For this reason, lead-acid batteries are not ideal for powering devices for a long period of time. Instead, they’re best for applications that need a short, powerful burst of energy.
Chemical Reaction for Discharging
When the battery is discharged, it acts as a galvanic cell and the following chemical reaction occurs.
Negative:
Pb(s) + HSO4– + H2O(l) –> 2e– + PbSO4(s) + H3O+(aq) (oxidation )
Positive:
PbO2(s) + HSO4–(aq) + 3H3O+(aq) + 2e– –> PbSO4(s) + 5H2O(l) (reduction)
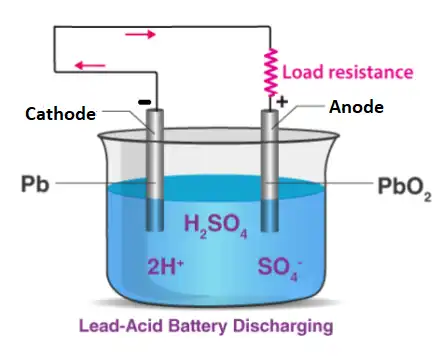
Lead sulfate is formed at both electrodes. Two electrons are also transferred in the complete reaction. The lead-acid battery is packed in a thick rubber or plastic case to prevent leakage of the corrosive sulphuric acid.
Lead Acid Batteries Charging
The sulphuric acid existing in the lead discharge battery decomposes and needs to be replaced. Sometimes, the plates change their structure by themselves. Eventually, the battery becomes less efficient and should be charged or changed.
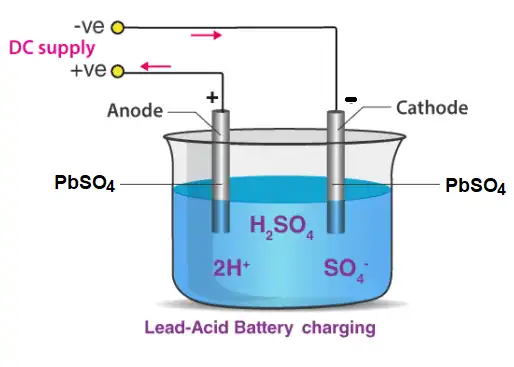
When car batteries spend considerable durations of time in their discharged states, the lead sulfate build-up may become extremely difficult to remove. This is the reason why lead-acid batteries must be charged as soon as possible (to prevent the building up of lead sulfate). Charging of the lead batteries is usually done by providing an external current source.
A plug is inserted which is linked to the lead-acid battery and the chemical reaction proceeds in the opposite direction. In cases where the sulphuric acid in the battery (or some other component of the battery) has undergone decomposition, the charging process may become inefficient. Therefore, it is advisable to check the battery periodically.
Chemical Reaction for Recharging
The chemical reaction that takes place when the lead-acid battery is recharging can be found below.
Negative:
2e– + PbSO4(s) + H3O+(aq) –> Pb(s) + HSO4– + H2O(l) (reduction)
Positive:
PbSO4(s) + 5H2O(l) –> PbO2(s) + HSO4–(aq) + 3H3O+(aq) + 2e– (oxidation)
While recharging, the automobile battery functions like an electrolytic cell. The energy required to drive the recharging comes from an external source, such as an engine of a car. It is also important to note that overcharging of the battery could result in the formation of by-products such as hydrogen gas and oxygen gas. These gases tend to escape from the battery, resulting in the loss of reactants.
The Self-Discharge of Lead-Acid Batteries
One unfortunate disadvantage of lead-acid batteries is that the chemical reaction described above can never be halted completely. In other words, these batteries will continue to discharge even when they’re not in use.
Normally, this self-discharge happens somewhat slowly, around 1% lost per day. But certain factors will increase this rate. For instance, the warmer the battery is, the faster it self-discharges.
Also, some devices use a little of the battery’s charge even when they’re turned off. The audio settings in your car are a good example of this. Your car radio uses battery power to “remember” these settings.
In any case, you’ll have to make sure you recharge your lead-acid batteries every once in a while or they will die.
The Death of Lead-Acid Batteries
So, what causes a lead-acid battery to die? Certain factors can damage or change the materials that are needed to cause the necessary chemical reaction. One such factor is allowing the battery to remain in a partially discharged state for too long.
Partial Discharge
As the battery discharges, it lowers the amount of electrolyte solution (the sulphuric acid mixed with water). This leaves the lead plates partially exposed.
If they remain exposed, the sulphate that is already bonded to the lead can harden. Then, it remains on the lead permanently, which decreases the battery’s ability to recharge.
This partial discharge is a common problem with car batteries. You see, the battery recharges when you drive. But if you don’t drive often, or you always make very short trips, your battery might never get fully recharged.
Deep Discharge
Another common cause of battery death is deep discharge. This is when your lead-acid battery is discharged below 50%.
When this happens, small pieces of the lead plates can actually break off and sink into the electrolyte solution. Then, there is less material available to cause the chemical reaction. If too much is broken off, the reaction won’t happen at all.
This is why your car battery becomes unusable if you accidentally leave the headlights on overnight. Even if you’re able to jump-start the dead battery, the damage has already been done. The battery is permanently ruined and will have to be replaced.
Overcharging
Overcharging happens when you keep charging a battery that’s already full. Doing this can break down the material of the electrolyte. Once this happens, there is no sulphate left to bond with the lead.
This is why you don’t want to keep a lead-acid battery plugged into a charger all the time. It’s better to only plug it in once in a while.
Pros and Cons of Lead Acid Batteries
Lead-acid batteries have powerful voltage for their size. Thus, they can power heavy-duty tools and equipment.
They can even power electric vehicles, like golf carts. However, in this case, you’d need to be careful to charge the battery often enough (and without overcharging it). If you don’t, the vehicle will die before reaching its destination, which will also damage the battery.
Additionally, lead-acid batteries are great for starting motor vehicles. They provide an intense jolt of energy to start the vehicle and then they recharge as the vehicle drives.
On the other hand, they are not good for devices you wish to use for long periods of time, like cell-phones. Also, they self-discharge when not in use, which will eventually kill the battery.
In other words, you can’t just leave them sitting around. Thus, they are a bad option for any application that will not be used frequently.
What Will You Use Lead-Acid Batteries For?
Now that your questions are answered, use this guide to determine if lead-acid batteries are the right choice for your needs. Also, do you know anyone else who’s wondering, “How does a lead-acid battery work?” If so, please share this guide with them.
Frequently Asked Questions – FAQs
What is in a lead-acid battery?
How a lead acid battery is made?
How do you maintain a lead-acid battery?
Is a lead-acid battery wet or dry?
What type of battery is lead-acid?