MATERIALS USED FOR LEAD ACID BATTERIES
The primary active materials required to construct lead acid batteries are:
- Lead peroxide (PbO2): Dark brown, hard and brittle substance to form the positive plate.
- Sponge lead (Pb): The pure lead in soft sponge conditions creates the negative plate.
- Dilute sulfuric acid (H2SO4): A strong acid and a good electrolyte. It is highly ionised, and most of the heat released in dilution comes from the hydration of the hydrogen ions. It is used for the lead acid battery with a ratio of water: acid = 3:1.
HOW DO LEAD ACID BATTERIES WORK?
The lead acid storage battery is formed by dipping the lead peroxide plate and sponge lead plate in dilute sulfuric acid. An electric current is connected externally between these plates. In diluted sulfuric acid, the acid molecules split into positive hydrogen ions (H+) and negative sulfate ions (SO4 − −). When it reaches the PbO2 plate, the hydrogen ions receive electrons from it and become hydrogen atom which again attacks PbO2 and forms PbO and H2O (water). This PbO reacts with H2 SO4 and forms PbSO4 and H2O (water).
SO4− − ions (anions) move towards the electrode (anode) connected with the positive terminal of the DC source, where they will give up their extra electrons and become radical SO4. This radical SO4 cannot exist alone; hence reacts with PbSO4 of anode and forms lead peroxide (PbO2) and sulfuric acid (H2SO4).
When Lead acid Batteries are Charged?
Charging is a process that reverses the electrochemical reaction. It converts the electrical energy from the charger into chemical energy in the battery. However, a battery does not store electricity; it keeps the chemical energy necessary to produce electricity.
A battery charger reverses the current flow, providing that the charger has a greater voltage than the battery. The charger creates an excess of electrons at the negative plates, and the positive hydrogen ions are attracted to them. The hydrogen reacts with the lead sulfate to form sulfuric acid and lead, and when most of the sulfate is gone, hydrogen rises from the negative plates. The oxygen in the water reacts with the lead sulfate on the positive plates to turn them again into lead dioxide, and oxygen bubbles rise from the positive plates when the reaction is almost complete. This is called gassing.

Sealed Lead Acid Batteries Types
The first sealed, or maintenance-free, lead acid emerged in the mid-1970s. Engineers argued that the term “sealed lead acid” was a misnomer because no lead acid battery can be totally sealed. To control venting during stressful charge and rapid discharge, valves have been added that release gases if pressure builds up. Rather than submerging the plates in a liquid, the electrolyte is impregnated into a moistened separator, a design that resembles nickel- and lithium-based systems. This enables operating the battery in any physical orientation without leakage.
The sealed battery contains less electrolyte than the flooded type, hence the term “acid-starved.” Perhaps the most significant advantage of sealed lead acid is the ability to combine oxygen and hydrogen to create water and prevent dry out during cycling. The recombination occurs at a moderate pressure of 0.14 bar (2psi). The valve serves as a safety vent if the gas buildup rises. Repeated venting should be avoided as this will lead to an eventual dry-out. According to RWTH, Aachen, Germany (2018), the cost of VRLA is about $260 per kWh.
Several types of sealed lead acid have emerged and the most common are gel, also known as valve-regulated lead acid (VRLA), and absorbent glass mat (AGM). The gel cell contains a silica type gel that suspends the electrolyte in a paste. Smaller packs with capacities of up to 30Ah are often called SLA (sealed lead acid). Packaged in a plastic container, these batteries are used for small UPS, emergency lighting and wheelchairs. Because of low price, dependable service and low maintenance, the SLA remains the preferred choice for healthcare in hospitals and retirement homes. The larger VRLA is used as power backup for cellular repeater towers, Internet hubs, banks, hospitals, airports and more.
The AGM suspends the electrolyte in a specially designed glass mat. This offers several advantages to lead acid systems, including faster charging and instant high load currents on demand. AGM works best as a mid-range battery with capacities of 30 to 100Ah and is less suited for large systems, such as UPS. Typical uses are starter batteries for motorcycles, start-stop function for micro-hybrid cars, as well as marine and RV that need some cycling.
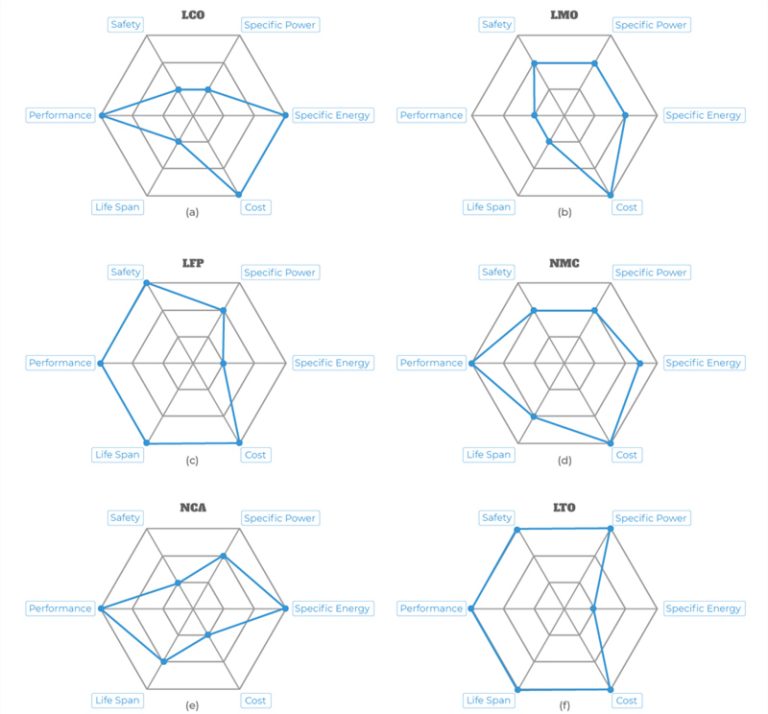
With cycling and age, the capacity of AGM fades gradually; gel, on the other hand, has a dome shaped performance curve and stays in the high performance range longer but then drops suddenly towards the end of life. AGM is more expensive than flooded, but is cheaper than gel. (Gel would be too expensive for start/stop use in cars.)
Starter Batteries
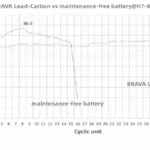
The starter battery is designed to crank an engine with a momentary high-power load lasting a second or so. For its size, the battery is able to deliver high current but it cannot be deep-cycled. Starter batteries are rated with Ah or RS (reserve capacity) to indicate energy storage capability, as well as CCA (cold cranking amps) to signify the current a battery can deliver at cold temperature. SAE J537 specifies 30 seconds of discharge at –18°C (0°F) at the rated CCA ampere without the battery voltage dropping below 7.2 volts. RC reflects the runtime in minutes at a steady discharge of 25.
Starter batteries have a very low internal resistance that is achieved by adding extra plates for maximum surface area. The plates are thin and the lead is applied in a sponge-like form that has the appearance of fine foam, expanding the surface area further. Plate thickness, which is important for a deep-cycle battery is less important because the discharge is short and the battery is recharged while driving; the emphasis is on power rather than capacity.
Deep-cycle Batteries
The deep-cycle battery is built to provide continuous power for wheelchairs, golf cars, forklifts and more. This battery is built for maximum capacity and a reasonably high cycle count. This is achieved by making the lead plates thick. Although the battery is designed for cycling, full discharges still induce stress and the cycle count relates to the depth-of-discharge (DoD). Deep-cycle batteries are marked in Ah or minutes of runtime. The capacity is typically rated as a 5-hour and 20-hour discharge.
A starter battery cannot be swapped with a deep-cycle battery or vice versa. While an inventive senior may be tempted to install a starter battery instead of the more expensive deep-cycle on his wheelchair to save money, the starter battery would not last because the thin sponge-like plates would quickly dissolve with repeated deep cycling.
There are combination starter/deep-cycle batteries available for trucks, buses, public safety and military vehicles, but these units are big and heavy. As a simple guideline, the heavier the battery is, the more lead it contains, and the longer it will last. Table 3 compares the typical life of starter and deep-cycle batteries when deep cycled.
|
Depth of Discharge
|
Starter Battery
|
Deep-Cycle Battery
|
|
100%
|
12–15 cycles
|
150–200 cycles
|
|
50%
|
100–120 cycles
|
400–500 cycles
|
|
30%
|
130–150 cycles
|
1,000 and more cycles
|
Table 3: Cycle performance of starter and deep-cycle batteries.
A discharge of 100% refers to a full discharge; 50% is half and 30% is a moderate discharge with 70% remaining.
Lead Acid batteries or Lithium-ion batteries in your Car?
Ever since Cadillac introduced the starter motor in 1912, lead acid batteries served well as battery of choice. Thomas Edison tried to replace lead acid with nickel-iron (NiFe), but lead acid prevailed because of its rugged and forgiving nature, as well as low cost. Now the lead acid serving as starter battery in vehicles is being challenged by Li-ion.
Figure 4 illustrates the characteristics of lead acid and Li-ion. Both chemistries perform similarly in cold cranking. Lead acid is slightly better in W/kg, but Li-ion delivers large improvements in cycle life, better specific energy in Wh/kg and good dynamic charge acceptance. Where Li-ion falls short is high cost per kWh, complex recycling and less stellar safety record than lead acid.
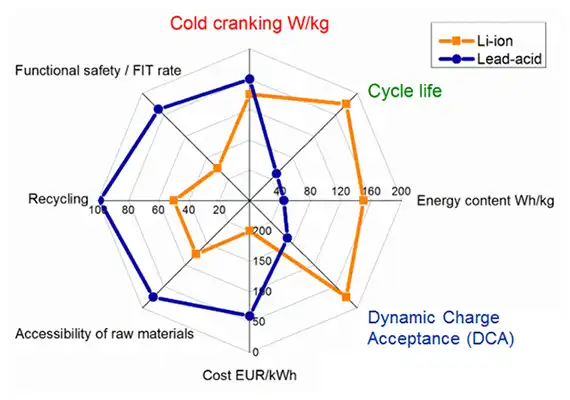
Lead is toxic and environmentalists would like to replace the lead acid battery with an alternative chemistry. Europe succeeded in keeping NiCd out of consumer products, and similar efforts are being made with the starter battery. The choices are NiMH and Li-ion, but the price is too high and low temperature performance is poor. With a 99 percent recycling rate, the lead acid battery poses little environmental hazard and will likely continue to be the battery of choice.
Table 5 lists advantages and limitations of common lead acid batteries in use today. The table does not include the new lead acid chemistries.
| Advantages |
|
| Limitations |
|
Table 5: Advantages and limitations of lead acid batteries.

Lithium-ion vs. lead acid batteries: who wins?
| LITHIUM-ION | LEAD ACID | |
|---|---|---|
| Cost | $5,000 – $15,000 | $500 – $1,000+ |
| Capacity | 15+ kWh | 1.5-5kWh |
| Depth of discharge | 85% | 50% |
| Efficiency | 95% | 80-85% |
| Lifespan | 10-15 years | 3-12 years |
lithium-ion vs lead acid batteries
Lithium-ion and lead acid batteries can both store energy effectively, but each has unique advantages and drawbacks. Here are some important comparison points to consider when deciding on a battery type:
Cost
The one category in which lead acid batteries seemingly outperform lithium-ion options is in their cost. A lead acid battery system may cost hundreds or thousands of dollars less than a similarly-sized lithium-ion setup – lithium-ion batteries currently cost anywhere from $5,000 to $15,000 including installation, and this range can go higher or lower depending on the size of system you need.
While lead acid batteries typically have lower purchase and installation costs compared to lithium-ion options, the lifetime value of a lithium-ion battery evens the scales. Below, we’ll outline other important features of each battery type to consider, and explain why these factors contribute to an overall higher value for lithium-ion battery systems.
Capacity
A battery’s capacity is a measure of how much energy can be stored (and eventually discharged) by the battery. While capacity numbers vary between battery models and manufacturers, lithium-ion battery technology has been well-proven to have a significantly higher energy density than lead acid batteries. This means that more energy can be stored in a lithium-ion battery using the same physical space. Because you can store more energy with lithium-ion technology, you can discharge more energy, thus powering more appliances for longer periods of time.
Depth of discharge
A battery’s depth of discharge is the percentage of the battery that can be safely drained of energy without damaging the battery. While it is normal to use 85 percent or more of a lithium-ion battery’s total capacity in a single cycle, lead acid batteries should not be discharged past roughly 50 percent, as doing so negatively impacts the lifetime of the battery. The superior depth of discharge possible with lithium-ion technology means that lithium-ion batteries have an even higher effective capacity than lead acid options, especially considering the higher energy density in lithium-ion technology mentioned above.
Efficiency
Just like solar panel efficiency, battery efficiency is an important metric to consider when comparing different options. Most lithium-ion batteries are 95 percent efficient or more, meaning that 95 percent or more of the energy stored in a lithium-ion battery is actually able to be used. Conversely, lead acid batteries see efficiencies closer to 80 to 85 percent. Higher efficiency batteries charge faster, and similarly to the depth of discharge, improved efficiency means a higher effective battery capacity.
Lifespan
Batteries are also similar to solar panels in that they degrade over time and become less effective as they age. Discharging a battery to power your home or appliances and then recharging it with solar energy or the grid counts as one “cycle”. The numbers vary from study to study, but lithium-ion batteries generally last for several times the number of cycles as lead acid batteries, leading to a longer effective lifespan for lithium-ion products.
Lead-Acid Batteries Lithium-ion Batteries