Lead Acid Battery
Definition: The lead acid battery which uses sponge lead and lead peroxide for the conversion of the chemical energy into electrical power, such type of battery is called a lead acid battery. The lead acid battery is most commonly used in the power stations and substations because it has higher cell voltage and lower cost.
Construction of Lead Acid Battery
The various parts of the lead acid battery are shown below. The container and the plates are the main part of the lead acid battery. The container stores chemical energy which is converted into electrical energy by the help of the plates.
1. Container – The container of the lead acid battery is made of glass, lead lined wood, ebonite, the hard rubber of bituminous compound, ceramic materials or moulded plastics and are seated at the top to avoid the discharge of electrolyte. At the bottom of the container, there are four ribs, on two of them rest the positive plate and the others support the negative plates.
The prism serves as the support for the plates and at the same time protect them from a short-circuit.The material of which the battery containers are made should be resistant to sulfuric acid, should not deform or porous, or contain impurities which damage the electrolyte.
2. Plate – The plate of the lead-acid cell is of diverse design and they all consist some form of a grid which is made up of lead and the active material. The grid is essential for conducting the electric current and for distributing the current equally on the active material. If the current is not uniformly distributed, then the active material will loosen and fall out.
 The grids are made up of an alloy of lead and antimony. These are usually made with the transverse rib that crosses the places at a right angle or diagonally. The grid for the positive and negative plates are of the same design, but the grids for the negative plates are made lighter because they are not as essential for the uniform conduction of the current.
The grids are made up of an alloy of lead and antimony. These are usually made with the transverse rib that crosses the places at a right angle or diagonally. The grid for the positive and negative plates are of the same design, but the grids for the negative plates are made lighter because they are not as essential for the uniform conduction of the current.
The plates of the battery are of two types. They are the formed plates or plante plates and pasted or faure plates.
Plante’s plates are used largely for stationary batteries as these are heavier in weight and more costly than the pasted plates. But the plates are more durable and less liable to lose active material by rapid charging and discharging. The plantes plate has low capacity weight-ratio.
Faure process is much suitable for manufacturing of negative plates rather than positive plates. The negative active material is quite tough, and it undergoes a comparatively low change from charging and discharging.
3. Active Material – The material in a cell which takes active participation in a chemical reaction (absorption or evolution of electrical energy) during charging or discharging is called the active material of the cell. The active elements of the lead acid are
- Lead peroxide (PbO2) – It forms the positive active material. The PbO2 are dark chocolate broom in colour.
- Sponge lead – Its form the negative active material. It is grey in colour.
- Dilute Sulfuric Acid (H2SO4) – It is used as an electrolyte. It contains 31% of sulfuric acid.
The lead peroxide and sponge lead, which form the negative and positive active materials have the little mechanical strength and therefore can be used alone.
4. Separators – The separators are thin sheets of non-conducting material made up of chemically treated leadwood, porous rubbers, or mats of glass fibre and are placed between the positive and negative to insulate them from each other. Separators are grooved vertically on one side and are smooth on the other side.
5. Battery Terminals – A battery has two terminals the positive and the negative. The positive terminal with a diameter of 17.5 mm at the top is slightly larger than the negative terminal which is 16 mm in diameter.
Working Principle of Lead Acid Battery
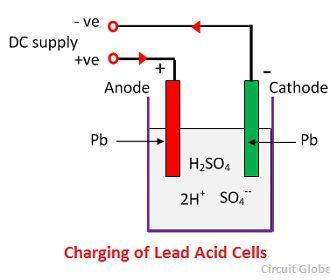
When the sulfuric acid dissolves, its molecules break up into positive hydrogen ions (2H+) and sulphate negative ions (SO4—) and move freely. If the two electrodes are immersed in solutions and connected to DC supply then the hydrogen ions being positively charged and moved towards the electrodes and connected to the negative terminal of the supply. The SO4— ions being negatively charged moved towards the electrodes connected to the positive terminal of the supply main (i.e., anode).
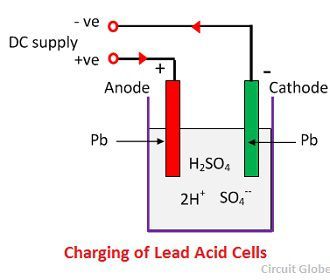 Each hydrogen ion takes one electron from the cathode, and each sulphates ions takes the two negative ions from the anodes and react with water and form sulfuric and hydrogen acid.
Each hydrogen ion takes one electron from the cathode, and each sulphates ions takes the two negative ions from the anodes and react with water and form sulfuric and hydrogen acid.
The oxygen, which produced from the above equation react with lead oxide and form lead peroxide (PbO2.) Thus, during charging the lead cathode remain as lead, but lead anode gets converted into lead peroxide, chocolate in colour.
If the DC source of supply is disconnected and if the voltmeter connects between the electrodes, it will show the potential difference between them. If wire connects the electrodes, then current will flow from the positive plate to the negative plate through external circuit i.e. the cell is capable of supplying electrical energy.
Chemical Action During Discharging
When the cell is full discharge, then the anode is of lead peroxide (PbO2) and a cathode is of metallic sponge lead (Pb). When the electrodes are connected through a resistance, the cell discharge and electrons flow in a direction opposite to that during charging.
The hydrogen ions move to the anode and reaching the anodes receive one electron from the anode and become hydrogen atom. The hydrogen atom comes in contacts with a PbO2, so it attacks and forms lead sulphate (PbSO4), whitish in colour and water according to the chemical equation.
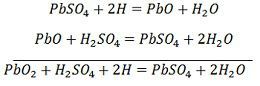
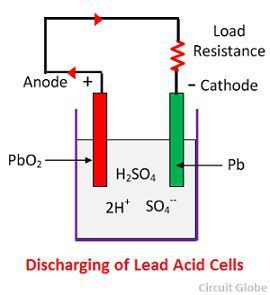 The each sulphate ion (SO4—) moves towards the cathode and reaching there gives up two electrons becomes radical SO4, attack the metallic lead cathode and form lead sulphate whitish in colour according to the chemical equation.
The each sulphate ion (SO4—) moves towards the cathode and reaching there gives up two electrons becomes radical SO4, attack the metallic lead cathode and form lead sulphate whitish in colour according to the chemical equation.
Chemical Action During Recharging
For recharging, the anode and cathode are connected to the positive and the negative terminal of the DC supply mains. The molecules of the sulfuric acid break up into ions of 2H+ and SO4—. The hydrogen ions being positively charged moved towards the cathodes and receive two electrons from there and form a hydrogen atom. The hydrogen atom reacts with lead sulphate cathode forming lead and sulfuric acid according to the chemical equation.
![]()
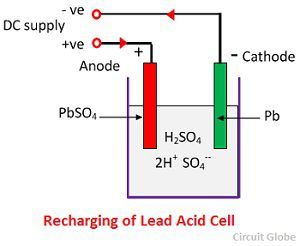 SO4— ion moves to the anode, gives up its two additional electrons becomes radical SO4, react with the lead sulphate anode and form leads peroxide and lead sulphuric acid according to the chemical equation.
SO4— ion moves to the anode, gives up its two additional electrons becomes radical SO4, react with the lead sulphate anode and form leads peroxide and lead sulphuric acid according to the chemical equation.![]() The charging and discharging are represented by a single reversible equation given below.
The charging and discharging are represented by a single reversible equation given below.
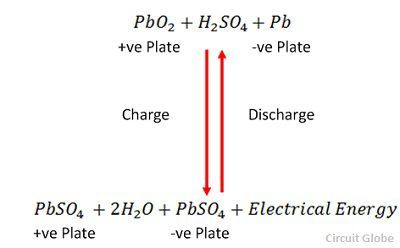 The equation should read downward for discharge and upward for recharge.
The equation should read downward for discharge and upward for recharge.
Lead-Acid Battery Charging Methods
The lead-acid battery stores chemical energy and this energy is converted into electrical energy whenever requires. The conversion of energy from chemical to electrical is known as the charging. And when the electric power changes into chemical energy then it is known as discharging of the battery. During the charging process, the current passes inside the battery because of chemical changes. The lead-acid battery mainly uses two types of charging methods namely the constant voltage charging and constant current charging.
Constant voltage Charging
It is the most common method of charging the lead acid battery. It reduces the charging time and increases the capacity up to 20%. But this method reduces the efficiency by approximately 10%.
In this method, the charging voltage is kept constant throughout the charging process. The charging current is high in the beginning when the battery is in the discharge condition. The current is gradually dropping off as the battery picks up charge resulting in increase back emf.
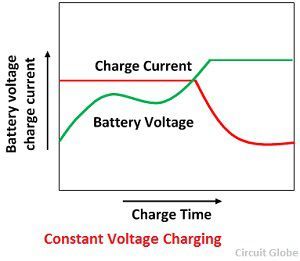 The advantages of charging at constant voltage are that it allows cells with different capacities and at the different degree of discharge to be charges. The large charging current at the beginning of the charge is of relatively short duration and will not harm the cell.
The advantages of charging at constant voltage are that it allows cells with different capacities and at the different degree of discharge to be charges. The large charging current at the beginning of the charge is of relatively short duration and will not harm the cell.
At the end of the charge, the charging current drops to almost zero because the voltage of the battery becomes nearly equal to the voltage of the supply circuit.
Constant Current Charging
In this method of charging the batteries are connected in series so as to form groups and each group charges from the DC supply mains through loading rheostats. The number of charging in each group depends on the charging circuit voltage which should not be less than the 2.7 V per cell.
The charging current is kept constant throughout the charging period by reducing the resistance in the circuit as the battery voltage goes up. In order of avoiding excessive gassing or overheating, the charging may be carried out in two steps. An initial charging of approximately higher current and a finishing rate of low current.
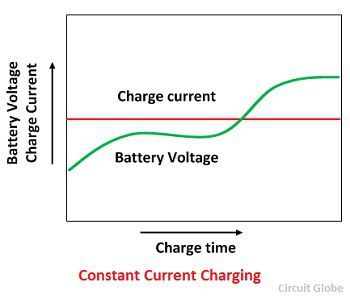 In this method, the charge current is approximately one-eighth of its ampere ratings. The excess voltage of the supply circuit is absorbed in the series resistance. The groups of the battery to be charged should be so connected that the series resistance consumes as little energy as possible.
In this method, the charge current is approximately one-eighth of its ampere ratings. The excess voltage of the supply circuit is absorbed in the series resistance. The groups of the battery to be charged should be so connected that the series resistance consumes as little energy as possible.
The current carrying capacity of series resistance should be greater than or equal to the required charging current otherwise, the resistance will overheat and burn out.
The group of batteries which is to be selected should have the same capacity. If the battery has a different capacity, then they will have to be set according to the least capacity.
Tips: more detail information,for Lead acid gel battery.